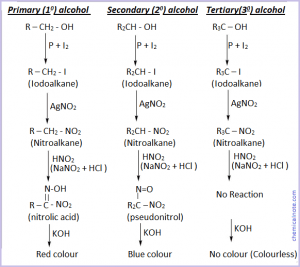
What are Primary (10) , Secondary (20) and Tertiary(30) alcohols ?
- Alcohol in which the carbon atom bonded with –OH group is further bonded with one or non other carbon atom is called primary alcohol. Eg. methanol, ethanol, etc.
- Alcohol in which the carbon atom bonded with –OH group is further bonded with two other carbon atoms is called secondary alcohol. Eg. propan-2-ol.
- Alcohol in which the carbon atom bonded with –OH group is further bonded with three other carbon atoms is called tertiary alcohol. Eg. 2-methyl propan-2-ol.

Victor Meyer’s method to distinguish alcohols :
In Victor Meyer’s method, alcohol is first treated with P and I2 to get iodoalkane , which is then treated with AgNO2 ( silver nitrate) to get nitroalkane. The nitroalkane thus obtained is treated with nitrous acid ( a mixture of NaNO2 and dil. HCl) and the resulting solution is finally made alkaline with KOH and the colour is observed. If the colour obtained is :
Red Colour = Primary alcohol
Blue Colour = Secondary alcohol
No colour = Tertiary alcohol.
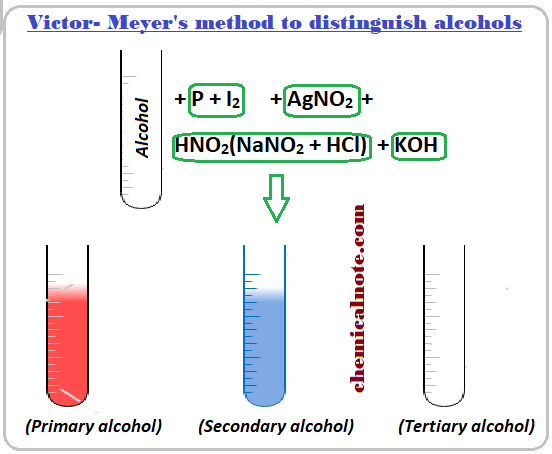
Note : Don’t be confused on third step i.e. reaction of nitroalkane with HNO2 ( HONO). Just try to remove water molecule as by-product.
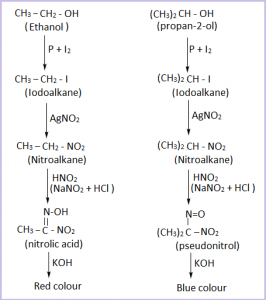
Q. How would you distinguish ethanol and propan-2- ol using Victor Meyer’s method?
→ Ethanol is a primary alcohol while propan-2-ol is a secondary alcohol.
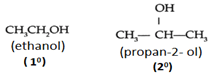
In Victor Meyer’s method, alcohol is first treated with P and I2 to get iodoalkane , which is then treated with AgNO2 ( silver nitrate) to get nitroalkane. The nitroalkane thus obtained is treated withnitrous acid ( a mixture of NaNO2 and dil. HCl) and the resulting solution is finally made alkaline with KOH and the colour is observed. If the colour obtained is :
Red Colour = Primary alcohol
Blue Colour = Secondary alcohol
Hence, ethanol and propan-2-ol can be distinguished by observing colour in Victor- Meyer’s method as ethanol gives red colour while propan-2-ol gives blue colour .