Phenol and Nomenclature of Phenol
By Anup Pokhrel
Phenols are hydroxy derivatives of benzene obtained by replacing one of the hydrogens of benzene by a hydroxy group. They are represented by general formula Ar-OH, where Ar is phenyl,substituted phenyl or some other aryl group (eg, naphthyl,phenanthryl, etc. When the hydroxyl group is bonded to a carbon of the side chain, ArCH2-OH, the compound is termed as aromatic alcohol.
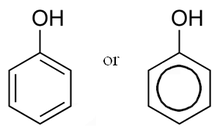
Phenol
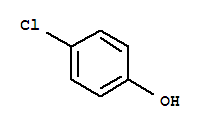
P-Chlorophenol
Phenols differ from alcohols in most of their properties and methods of preparation. However, both being hydroxyl compounds resembles each other to a limited extent, eg ., both can be converted into esters
Like alcohols, phenols may also be classified as monohydric, dihydric,trihydric etc. depending upon the number of hydroxyl groups attached to the aromatic ring.
Nomenclature of phenol.
Phenols are generally named as derivatives of the simplest member of the family,i,e phenol. In the case of phenols having only one additional substituent, the relative position is usually indicated by letters o-(for 1,2-) m- (for(1,3-) and P-for (1,4-). However, numerals are used if more than two substituents are present on the ring. For example.
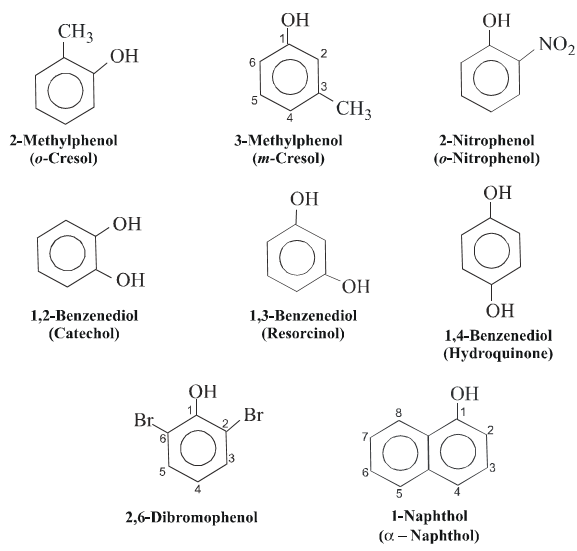 v
v
Inductive effect.
I think when a more electronegative atom or group is attached to the end of a carbon chain (especially in saturated hydrocarbon) , the shared or bonded electrons are displaced towards more electronegative atom or group. This cause the development of a certain degree of polarity in the bond or molecule. Consequently, the more electronegative atom acquires a small (small) negative charge (Δ–) while less electronegative atom acquires a slight positive( Δ+ ) charge. This is, in fact, due to the shifting of (σ) electrons along a saturated carbon chain. This effect is known as defined as the polarity developed in a molecule by displacement of bonding electrons along a carbon chain as a result of higher electronegativity of one atom compare to another.
Consider chloropropane
In chloropropane, more electronegative ‘Cl’ atom is attached to one end of the carbon chain. Due to the more electronegativity of Cl atom (σ) electrons of C-Cl bonds shifts to Cl atoms . Due to this, Cl atom acquires a small negative charge (Δ–) and adjacent C-atom acquires a small positive charge ( Δ+). This effect is further transmitted along a chain of C atom but the magnitude of the effect decreases with the increasing distance from the substituent or more electronegative atom or groups. Thus this effect is observed more in C1 then C2 and C3.
Therefore, inductive effect is permanent effect and involves (σ) electrons. Zero inductive effect is assumed in carbon-hydrogen bond.
$$C^3
Electrons donating or releasing groups (alkyl groups (CH3)3C, groups (No2, F, Cl, Br, I, OH, C6H5) produce -I effect.